There are several general types of beneficial bacteria involved in oxidizing ammonia to relatively safe nitrate in the freshwater aquarium. Scientists call these organisms “nitrifying” bacteria and archaea. We will use the term “beneficial bacteria” for both the nitrifying bacteria and the nitrifying archaea. Below in this article, we try to explain the complex science of “beneficial bacteria”.
This information is not necessary for setting up new aquariums or running existing aquariums. But some like to delve into the science. Read on if you are so inclined. Just be prepared for a very boring, long-winded dissertation only true nerds such as the author will be interested in.

Beneficial Bacteria in Depth
From a pure science perspective there are a whole series of “beneficial bacteria”, with more being discovered every year.
The sequential steps of this respiratory process are carried out by distinct microbial guilds, including ammonia-oxidizing bacteria (AOB) and archaea (AOA), nitrite-oxidizing bacteria (NOB), and newly discovered members of the genus Nitrospira that conduct complete ammonia oxidation (comammox). Even though all of these nitrifiers have been identified within water treatment systems, their relative contributions to nitrogen cycling are poorly understood. Although AOA contribute to nitrification in many wastewater treatment plants, they are generally outnumbered by AOB. In contrast, AOA and comammox Nitrospira typically dominate relatively low ammonia environments such as drinking water treatment, tertiary wastewater treatment systems, and aquaculture/aquarium filtration.
“Ammonia-oxidizing archaea and complete ammonia-oxidizing Nitrospira in water treatment systems”, Al-Ajeel 2022:
“Beneficial bacteria” are responsible for what is known as the nitrogen cycle in an aquarium. These bacteria ONLY grow and reproduce on surfaces, they do NOT grow or reproduce floating free in the aquarium water (that is a very old aquarium myth). Some of these “beneficial bacteria” do this oxidation of ammonia in two steps. The first step is to oxidize ammonia NH₃ to nitrite NO₂ (AOB and AOA). Nitrite NO₂ is then oxidized to nitrate NO₃ (NOB), which is much less toxic than either ammonia or nitrite. Some bacteria do this oxidation in a single step (comammox Nitrospira).
Because these chemical processes are very low energy reactions these bacteria need a lot of molecules of all the chemicals to metabolize. They require something called “turbulent water flow” to get the chemicals to the bacterial surfaces in quantity. All the “beneficial bacteria” involved in this ammonia oxidation need amounts of oxygen (greater than 80% water saturation), carbon dioxide, and ammonia to thrive. They also all need a surface to hold on to, none can reproduce in a free-swimming mode. They are all very slow to multiply, with NO exceptions.

These “beneficial bacteria” are what are termed “obligative autotrophs” or “obligative chemotrophs”. What this means is that these bacteria cannot feed on “normal” food, namely proteins and carbohydrates. These bacteria MUST (note the term obligative!) feed on chemicals (this is the term “chemotrophs”), namely three things: ammonia gas NH₃, carbonate CO₃, and oxygen O₂. They use these chemicals to make up the proteins and ingredients in their bodies.
Because these bacteria feed on these three chemicals anything which increases the amount of all three chemicals will increase the rate at which the beneficial bacteria multiply.
So the required cycle time of an aquarium can theoretically be decreased by:
- Adding more ammonia NH₃
- Adding more carbonates CO₃
- Adding more oxygen O₂

But the effect of adding more of any of these is much much less than popular “science” says. Most research on beneficial bacteria is based on sewage treatment. Sewage treatment is just worlds different than oxidized ammonia in an aquarium. For instance, many tote the benefits of bio wheels on filters. Bio wheels are based on the use of circular aeration disks in sewage treatment. The claim is that because oxygen is important in ammonia oxidation one needs a large amount of oxygen a wheel provides.
But even during cycling, where one might be adding 2 ppm per day of ammonia, one only needs 2×3.6 = 7 ppm oxygen to oxidize the ammonia in one day. 7/24 = 0.3 ppm per hour. Any decent aeration will add 1 to 10 ppm oxygen per hour to an aquarium. So in aquarium oxygen is NOT a limiting factor and adding more with something like a biowheel doesn’t help very much. In a sewage treatment plant, the ammonia can be 40 ppm with a residence time in hours, a much different situation. That being said, we do advise one have VERY heavy aeration during cycling.

Because beneficial bacteria need a flow rate above what is found inside most aquariums, studies (“Temporal and Spatial Stability of Ammonia-Oxidizing Archaea and Bacteria in Aquarium Biofilters”, Bagchi et. al., 2014) have shown that 80% to 84% of the beneficial bacteria in an aquarium reside in the filter media. The substrate, aquarium walls, and ornaments have only a 16% to 20% contribution to ammonia oxidation and clear water. And in a very well filtered aquarium with crystal clear water this will be even more lopsided, more like 95% – 5%.
But remember Mother Nature is quite flexible. It is possible to force the substrate and the ornaments in an aquarium to do the biofiltration. If one does something like only using sponge filters in an aquarium and rinsing those sponge filters out thoroughly under running water once a week, one will force the substrate and ornaments to become the biofilter. This is quite acceptable way to do it. But do not try this with a heavy stocking.

Myths About Beneficial Bacteria
There are several very strongly held myths about beneficial bacteria constantly parroted in social media.
These are:
- #1 Beneficial Bacteria are the Only Beneficial Organisms in a Filter
- #2 Beneficial Bacteria Die Rapidly Without Food
- #3 High Nitrite Inhibits Growth
- #4 High Ammonia Levels Inhibit Growth
- #5 There are Special Strains of Beneficial Bacteria
- #6 Beneficial Bacteria Stop Reproducing at the end of cycling
- #7 Adding a lot of new fish to a long-established filter will overwhelm a limited amount of beneficial bacteria
- #8 “Bacterial Blooms” are beneficial bacteria
ALL these myths are simply not true!
We will examine these myths in more depth below.

Myth #1: Beneficial Bacteria are the Only Beneficial Organisms in a Filter
Well meaning but ill-informed individuals on social media say that “beneficial bacteria”, i.e. nitrifying bacteria that oxidize ammonia, are the only organisms of importance in a biofilm. They say that one ONLY needs sufficient surface area in a filter to remove the ammonia from the water. But there are many beneficial organisms beyond beneficial nitrifying bacteria in an aquarium.
The brown filter sludge in a filter is for the most part alive and not simply waste. Removing this mud does more harm than good. The purpose of the filter media is not to filter out particles from the water as is often assumed. The media serves as the habitat for a vast array of microorganisms that include bacteria, archaea, worms, ciliates, flagellates, and many others. These microorganisms live in a community that is based on biofilms. The biofilms are created by bacteria that secret extracellular polymeric substance (EPS), which is often called “slime”. The community forms a bioreactor that processes the waste and turns it into food and energy for its members, and ultimately into organic or inorganic products that are then used by plants, evaporate, or removed by water changes. It takes a considerable amount of time to establish this “filter community”; consequently, it is very important not to disturb it unless necessary.
Swiss Tropicals
This is probably the most intelligent statement any supplier of aquarium products has ever made.

As the Poret write-up alludes to, there are a whole host of beneficial organisms in most biofilms in the aquarium filter. These beneficial organisms continue to grow and reproduce well after a filter meets the technical definition of “cycled”.
These statements that nitrifying bacteria are the only important organisms miss the point that over filtration is the key to crystal clear water and healthy fish as it removes bacteria and dissolved organic compounds from the water. The heterotrophic bacteria in the brown gunk in a filter remove dissolved organic compounds from the water. This is very important to the health of the fish.
And there are a whole host of rotifers and ciliates which eat the bacteria in the water column as the bacteria flow over them from the aquarium. This reduction in bacteria in the water column gives crystal clear water and, more importantly, very healthy fish. Crystal clear water requires very roughly twenty times more surface area than ammonia oxidation.
Here is an interesting chart:
| Water contaminant | Biofiltration | Mechanical filtration | Most chemical filtration | Ultraviolet |
|---|---|---|---|---|
| Ammonia and nitrite | Removes | |||
| Bad bacteria | Removes | Removes | ||
| Pathogens like ich | Removes | Removes | ||
| Dissolved organics | Removes | |||
| Feces, uneaten food | Removes | Removes | ||
| Floating algae | Removes | Removes | Removes | |
| Tannic acid, dyes, smells | Removes |
Biofiltration is obviously easily 80% of the game per this chart.
As mentioned above “beneficial bacteria” or “nitrifying bacteria” are autotrophic organisms that feed on chemical like ammonia and nitrite. The other major category of organisms are heterotrophic organisms, which feeds on carbohydrates, proteins, plant matter and animal matter. What is interesting is to break the chart above down even further:
| Water contaminant | Autotrophic Biofiltration | Heterotrophic Biofiltration |
|---|---|---|
| Ammonia and nitrite | Removes | |
| Bad bacteria | Removes | |
| Pathogens like ich | Removes | |
| Dissolved organics | Removes | |
| Feces, uneaten food | Removes | |
| Floating algae | Removes | |
| Tannic acid, dyes, smells |
This give one a sense of how important heterotrophic organisms are in the filter.

Myth #2 Beneficial Bacteria Die Rapidly Without Food
There is a myth promulgated by many well meaning but ill-informed individuals on social media that the “beneficial bacteria” die if they don’t have food at least once a day. This isn’t true, the “beneficial bacteria” will live for several months to many years without food. Reference “Strategies of Aerobic ammonia-oxidizing Bacteria for Coping with Nutrient and Oxygen Fluctuations”, Geets et. al. 2006:
Nitrosomonas europaea cells starved for weeks, months, or even almost a year of ammonium were able to regain their ammonia‐oxidizing activity within minutes in batch and retentostat experiments
Wilhelm et al., 1998; Tappe et al., 1999; Laanbroek & Bär‐Gilissen, 2002:
In the book “The Isolation and Study of Nitrifying Bacteria”, W. Gibbs, 1919, found that BB or beneficial bacteria (scientifically these are “aerobic nitrifying bacteria”) retained their potency sealed in a bottle with air and moisture in it for seven years. BB is a very primitive, aerobic, low metabolism organism. BB does not die in months or years without food. They only need to be kept wet and open to the air.

It is important to note that Gibbs put moist non-organic soil in a bottle with a lot of air. The amount of air to the amount of organic material is important. If there was a small amount of air and a large amount of organics the oxygen levels would have dropped below what the beneficial bacteria can survive. In Gibbs bottle, they would have perished if this were the case.
Note that while drying beneficial bacteria does seem to reduce their effectiveness, there is anecdotal evidence drying does not kill beneficial bacteria. Many have reported very rapid cycle times when ornaments or gravel which haven’t been wet for months are used in a new aquarium.
If a canister filter has the flow stopped it can in some instances “go bad” (the bad smell of rotten food) in several hours to several days due to depletion of the oxygen in the water by large amounts of organic matter. Toxins made by bacteria at low oxygen levels will kill beneficial bacteria. So, canisters need to be opened and exposed to air if they must be shut down.

Myth #3: High Nitrite Inhibits Growth
Another myth about beneficial bacteria is that their growth is inhibited by nitrite levels over 9 ppm. This myth came about by a misunderstanding of the term “preservative”. Sodium nitrite is added to some foods (I.e. salami and pepperoni) as a “preservative” at a level of 9 ppm. Someone in the distant past saw this and assumed the sodium nitrite stopped bacterial growth. Therefore 9 ppm of nitrite in an aquarium would stop the growth of beneficial bacteria.
Sodium nitrite “preservative” in cured meats is there to “preserve” the color. It does nothing to the bacteria. Nitrite combines with the hemoglobin in the blood of the meat and forms a stable red color. The cured meat thus doesn’t turn gray.
In actuality, the optimum level of nitrite is roughly 200 ppm for cultivating beneficial bacteria per Gibbs 1919, Lewis 1958, Olah 1993, Tappe 1996, Willke 1996, Du 2003, Grzesiak 2017, and Kasmurik 2018.

Myth #4 High Ammonia Levels Inhibit Growth
Ammonia has a similar myth. One web source said:
Again, pay attention to the ammonia levels. Levels over 5 ppm can poison the nitrite-oxidizer bacteria. Destroying this colony of bacteria will prolong the cycling process.
This well intentioned web source was simply wrong. In actuality, the optimum level of ammonia is about 400 ppm for cultivating all strains of beneficial bacteria per Gibbs 1919, Lewis 1958, Olah 1993, Tappe 1996, Willke 1996, Du 2003, Grzesiak 2017, and Kasmurik 2018.
Most strains of nitrifying bacteria grow optimally at substrate concentrations of 1- 25 mM.
“The Family Nitrobacteraceae”, Watson et. al. 1981:
1- 25 mM of ammonia is 16 to 400 ppm, the optimum ammonia concentration per Watson.
Dr. Tim says not to exceed 5 ppm of ammonia because that will inhibit the growth of beneficial bacteria. Dr. Tim bases this claim on a “paper” he co-authored which identified different strains of beneficial bacteria that prospered in low ammonia concentrations rather than high ammonia concentrations. (“Identification of Bacteria Responsible for Ammonia Oxidation in Freshwater Aquaria”, Timothy A. Hovanec, et. al. 2001). This paper said that bioreactors culturing beneficial bacteria at a concentration of 5 to 10 ppm gave a different species profile of bacteria that reactors kept at 40 to 60 ppm ammonia.

The “paper” was done by a private, profit-oriented company (This is ALWAYS a red flag for me!) and had many obvious shortcomings. The paper notably did NOT test any intermediate levels (i.e. 10 to 40). It also did not test whether or not the 40 to 60 beneficial bacteria were unsuccessful at oxidizing ammonia in the 0 to 5 ppm range. So using this paper as the basis for saying “5 ppm ammonia inhibits beneficial bacterial growth” is simply incorrect.
Inhibition of nitrification by ammonia or nitrite is generally is not a problem in the treatment of domestic wastewater
“Inhibition of Nitrification by Ammonia and Nitrous Oxide”, Anthonisen et. al., 1976:
Considering that domestic wastewater ammonia levels hit 40 ppm and nitrite can hit 80 ppm, much higher than will be seen in the aquarium, ammonia or nitrite levels seen in the aquarium will not inhibit nitrification.

To test this claim by Dr. Tim about ammonia a test was run. This is the test:
Abstract
A test was run to see what ammonia additions are best for cycling an aquarium. The test showed one thing:
- The test showed using Dr. Tim’s regimen of only adding ammonia when the level hits 0.5 increased the time to cycle by 11 days over adding ammonia at 2 ppm per day.
Note that this test was deliberately kept quite simple so that anyone can easily duplicate it if they want to. A rigorous scientific test under laboratory conditions cannot be replicated by any hobbyist so that was not done. This test was not designed to be published in some scientific journals. But the huge difference in the test results makes the conclusions unmistakable and very valid, even if the science is not as rigorous as some would like.

Test Equipment:
- Home Depot five gallon (20 liter) buckets
- 100 grams BASF ammonium chloride dissolved in one gallon (3,785 liter) of distilled water
- air pumps and airlines
- mini sponge filters
Test Procedure:
6 buckets were set up with reverse osmosis water. A pinch of potassium phosphate was added to each bucket as earlier tests had shown that this addition is necessary if one is using ammonia additions only. The water was naturally about 6.8 in pH 0 KH after sitting for a week to stabilize.
- Three buckets had nothing done to them (the “controls”) with 2 ppm ammonia added daily. The buckets hit 14, 18, and 20 ppm ammonia, tested by dilution techniques.
- Three buckets had 6.8 pH and ammonia added per the directions of Dr. Tim, i.e. only add ammonia to the 2 ppm level when the ammonia level drops to 0.5 or lower.
The buckets were then cycled. The buckets were then tested every day for ammonia and nitrite.

Results:
Defining zero (technically it is less than or equal to 0.25 ppm) ammonia and nitrite for two days (two readings) as being cycled, the following results were obtained:
| Treatment | Average | Tank 1 | Tank 2 | Tank 3 |
|---|---|---|---|---|
| 2 ppm ammonia daily | 25 | 32 | 20 | 24 |
| Ammonia when 0.25 is hit (Dr. Tim’s) | 36 | 40 | 33 | 36 |
This test showed that Dr. Tim’s regimen of ammonia additions produced a cycle time that averaged 11 days more than adding 2 ppm daily of ammonia. This is not surprising. Beneficial bacteria, like all primitive organisms, reproduce at a rate directly proportional to the food supply. Dr. Tim’s regimen means roughly 1.1 ppm of ammonia is in the tank on the average while the 2 ppm regimen insures roughly 6 to 8 ppm is in the tank on the average. So the 2 ppm regimen goes faster.

Myth #5 There are Special Strains of Beneficial Bacteria
As usual, some will question these eight research articles by eight PhDs on the maximum levels of ammonia and nitrite that should be in an aquarium during cycling. They will say that these researchers didn’t use the type of beneficial bacteria found in the aquarium. That the aquarium species of beneficial bacteria need lower concentrations.
First off, all species are pretty well ubiquitous and found everywhere. The same species are found in aquariums, ponds, soil, and sewage treatment plants. The exact mix of species will vary by ammonia concentration and water chemistry but there will always be a lot of species that can do ammonia oxidation.
Secondly, the inoculant for the media in virtually all these university studies is soil. Soil contains only very minute amounts of ammonia and nitrite, well below 1 ppm. So the beneficial bacteria being cultivated by the universities are the ones living and reproducing at very low concentrations of ammonia.
And many of the manufacturers of “bacteria-in-a-bottle” claim to be cultivated “special strains” of nitrifying bacteria. This is just hogwash. It is very difficult to cultivate any one strain or several strains of any given nitrifying bacteria. You can buy small amounts of freeze-dried pure cultures of nitrifying bacteria for $200 to $400. These pure cultures will not be in a $15 bottle of bacteria.

Myth #6, Beneficial Bacteria Stop Reproducing at a Certain Point
Another myth for beneficial bacteria is the concept that beneficial bacteria stop growing when they reach a certain food loading. One well meaning but ill-informed social media commentator made the following statement that on the face of it might seem to make sense:
Having too much filtration is actually a waste. The nitrifying bacteria, commonly referred to as “BB” or beneficial bacteria colony will only grow to the size that matches it’s food source which is the Bio-load… The bio-load obviously comes from the fish waste and any regular extra food left uneaten (which there shouldn’t be if one is feeding their fish correctly). The BB colony will grow large enough only to consume the amount of waste being produced. No more, no less. So over-filtration is technically not possible.

This explanation goes sideways because it contains a fundamental misunderstanding about how bacteria live and multiply. Think of it this way. Beneficial bacteria take in food and multiply by dividing in two. So with a large feed of ammonia during cycling the bacteria will multiply at say once every 24 hours. They will multiply every 24 hours till they reach a level of say 1 billion at the end of cycling an aquarium when the ammonia level is say 100 parts per billion (i.e. undetectable on the API test).
If the feed of ammonia stopped cold at the end of cycling the beneficial bacteria would stop reproducing. But the ammonia feed does not stop at the end of cycling, the fish keep adding ammonia. So the beneficial bacteria keep reproducing, only at a slower rate. The 1 billion might then become 2 billion in two weeks, 4 billion at eight weeks, 8 billion at twenty weeks, etc. So in twenty weeks, without increasing the bioload, the capacity of the biofilter will go up by a factor of eight, assuming one doesn’t clean the filter. Of course this is idealized and very simplified.

Lower order organisms like bacteria do not require a given amount of food per day to survive or reproduce. For example, an autotrophic bacteria supplied with 0.4 ppm ammonia per day from fish may well split in two every day (24 hours). At 0.25 ppm the aquarist says his tank is now cycled and ready to go. But the fish continue to put out ammonia and the bacteria continue to feed. At 0.1 ppm ammonia the autotrophic bacteria might split every 4 days. At 0.05 ppm it might split every 8 days. And at 0.025 ppm it might split in two every 16 days.
So the NUMBER of bacteria in a system can continue to climb well past the point required to produce ammonia levels below 0.25 ppm. The lower the amount of food the slower the bacteria reproduce but they don’t stop reproducing completely till the level of ammonia hits true zero. Since fish constantly excrete ammonia this level of ammonia cannot hit true zero in any tank.
So a well established aquarium (greater than four months old without filter media cleaning) can double or even quadruple the number of fish in the aquarium and not see a spike in ammonia. Since the reproduction rate is being reduced exponentially and there are organisms eating the beneficial bacteria in the filter media, there is a practical limit to the growth but it is probably on the order of five to ten times the number of bacteria required to get the ammonia level below 0.25 ppm.

Myth #7 Adding a lot of new fish to a long-established filter will overwhelm a limited amount of beneficial bacteria
So a well-established aquarium (greater than four months old without filter media cleaning) can double or even quadruple the number of fish in the aquarium and not see a spike in ammonia. Since the reproduction rate is a logarithmic curve and organisms are eating the beneficial bacteria in the filter media, there is a practical limit to the growth but it is probably on the order of three to ten times the number of bacteria required to get the ammonia level below 0.25 ppm.

Myth #8 “Bacterial Blooms” are Beneficial Bacteria
Just when the author thinks he has heard all the myths there are another one pops up. A YouTube video recently came out where the presenter had done a “ton of research” and had “surprisingly found” that beneficial bacteria supposedly can multiply and live in profusion in aquarium water. Supposedly, according to this well meaning presenter, a “bacterial bloom” is a bloom of beneficial nitrifying bacteria.
Can’t sugarcoat it. The presenter is just plain wrong. As outlined above, beneficial bacteria can only capture enough ammonia and nitrite to reproduce when they are sitting on a surface. A “bacterial bloom” is a bloom of a whole host of tiny critters called “infusoria”. This “infusoria” (paramecium, rotifers, stentors, etc.) feed on something called “heterotrophic” bacteria which in turn feed on dissolved food and feces in the water column.
“Heterotrophic” is just a big word that means “eats normal food”. “Beneficial” bacteria are “obligative chemotrophs”, which means they eat the chemicals ammonia and nitrite and ONLY the chemicals ammonia and nitrite. Heterotrophic bacteria can reproduce every fifteen minutes while autotrophic bacteria can take 24 hours to reproduce. So in the period of a few days, one can have billions of heterotrophic bacteria and only a few thousand autotrophic bacteria. So even if beneficial bacteria could somehow grow suspended in water, there wouldn’t be very many of them compared to heterotrophic bacteria.

Upper Limits for Nitrite and Ammonia
So what are the upper limits for nitrifying bacteria for Nitrite and Ammonia? A reference is in order:
The ammonia and nitrite-oxidizing consortia developed here are unique systems, which oxidize ammonia and nitrite and have not been developed elsewhere. For the mass production of nitrifying consortia in a fermenter and also to obtain the highest level of activity in bioreactors, the optimum growth requirements such as substrate concentration, pH, temperature, and rate of airflow were determined. Accordingly, there is total inhibition of nitrification at above 2.75 grams per liter NH₄ and 7.5 grams per liter NO₂ in the test media 1 and 2.
“Optimum Growth Requirements of Nitrifying Consortia”, Ramachandran & Singh, 2004:
What this means is that the upper limit for ammonia is 2,750 ppm and the upper limit for nitrite is 7,500 ppm. There’s little chance this will be reached in an aquarium.

pH and Beneficial Bacteria
The water’s pH is also important to optimize the growth of beneficial bacteria.
“Most strains of nitrifying bacteria grow optimally at substrate concentrations of 1- 25 mM, a pH between 7.5 and 8.0, and a temperature of 25-30°C. However, many environments with suboptimal conditions still support the growth of nitrifying bacteria. For example, nitrifying bacteria are strict aerobes, yet they can be isolated from sewage-disposal aeration aquariums that are extremely low in oxygen. They can also be isolated from soils with a pH of 4 but cannot grow at a pH less than 6.”
“The Family Nitrobacteraceae”, Watson et. al. 1981:
Nitrifying bacteria, i.e. beneficial bacteria, grow best and a pH of 7.5 to 8.0. They don’t grow at a pH of 6.0. The work of Ramachandran & Singh 2004 confirmed this pH requirement.
For example, rates of nitrification and, in particular, ammonia oxidation in soil are significantly reduced in acid soils (de Boer and Kowalchuk, 2001), and significant batch growth of pure cultures of ammonia-oxidizing bacteria in liquid growth media does not occur below pH 7 (deBoer and Laanbroek, 1989; Allison and Prosser, 1991; Jiang and Bakken, 1999). The generally accepted explanation for reduced growth and activity of ammonia oxidizers at low pH is the exponential reduction in NH₃ availability with decreasing pH, through ionization to NH₄+(Frijlink et al., 1992), decreasing NH₃ diffusion and increasing the requirement for energy-dependent transport of NH₄+.
The Influence of Soil pH on the Diversity, Abundance and Transcriptional Activity of Ammonia Oxidizing Archaea and Bacteria”, Nicol et al, 2008:

Haug and McCarty (1972) observed a dramatic reduction of nitrification in a fixed-film system below pH 6 and cessation of nitrification at pH 5.5. Grady and Lim 1980 found the following relationships:
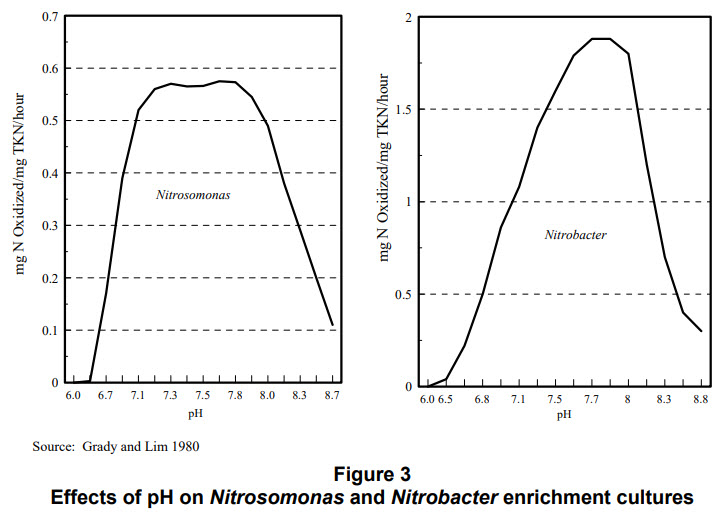
This chart makes it clear beneficial bacteria cease operating below 6.5 pH and above 8.7 pH.
Another reference is:
Below a pH of 6.8 the nitrifying bacteria are inhibited and do not remove toxic nitrogen wastes.
“Recirculating Aquaculture Tank Production Systems”, Masser et. al. Southern Regional Aquaculture Center, March 1992:
The reason for this pH requirement is simple. Nitrifying bacteria feed on gaseous unionized ammonia, i.e. NH₃. Ammonium, i.e. NH₄, can’t be used by beneficial bacteria as food. As any ammonium-ammonia solution goes down in pH the amount of gaseous ammonia decreases radically. It is virtually non-existent below 6.0 pH. So the beneficial bacteria have no more food available and stop reproducing.
Nitrifying bacteria also feed on carbon dioxide gas. At higher pHs, there is little carbon dioxide gas. So pHs above 8.6 can also significantly impede cycling.

Test Abstract:
A test was run to see what type of water and what ammonia additions are best for cycling an aquarium. The test showed one thing:
- The test showed that a pH of 6.0 stopped cycling cold.
Note that this test was deliberately kept quite simple so that anyone can easily duplicate it if they want to. A rigorous scientific test under laboratory conditions cannot be replicated by any hobbyist so that was not done. This test was not designed to be published in some scientific journals. But the huge difference in the test results makes the conclusions unmistakable and very valid, even if the science is not as rigorous as some would like.

Test Equipment:
- Home Depot five gallon (20 liter) buckets
- 100 grams BASF ammonium chloride dissolved in one gallon (3,785 liter) of distilled water
- air pump and airlines
- mini sponge filters
Test Procedure:
Six buckets were set up with reverse osmosis water. A pinch of potassium phosphate was added to each bucket as earlier tests indicated this was important if cycling with only ammonia. The water was naturally about 6.8 in pH 0 KH after sitting for a week to stabilize.
- Three buckets had nothing done to them (the “controls”) with 2 ppm ammonia added daily.
- Three buckets were taken down in pH to 6.0 with hydrochloric acid with 2 ppm ammonia added daily.
The buckets were then cycled. The buckets were then tested every day for ammonia and nitrite.

Results:
Defining zero (technically it is less than or equal to 0.25 ppm) ammonia and nitrite for two days (two readings) as being cycled, the following results were obtained:
| Treatment | Average | Tank 1 | Tank 2 | Tank 3 |
|---|---|---|---|---|
| 6,8 pH, 0 KH | 25 | 32 | 20 | 24 |
| 6,0 pH 0KH | >70 | >70 | >70 | >70 |
This test clearly shows that a low pH stops cycling cold.

There is a type of nitrifying organism called archaea which can operate at a very low pH. The problem is that these organisms are what are called obligative acidophiles. I.e. they ONLY work at low pH. These organisms also grow very slowly. A nitrifying bacteria might double in population every 24 hours under optimum conditions. Archaea will double in population every ten days under optimum conditions.
Since this is a logarithmic relationship this is a huge difference. And there is no good way to increase the speed with which they grow. So an acid tank will need four to twenty-four months to “cycle”.
So if one has an acid tank with low pH water one can simply cycle for four to twenty-four months and the aquarium will then cycle ammonia, just like an aquarium above 6.5 pH.

Beneficial Bacteria need Carbonate
Carbonate hardness (KH) is important for nitrifying bacteria in the aquarium. Nitrifying bacteria in sewage treatment plants need calcium carbonate (limestone) additions. These bacteria use the carbonate to prevent the sewage from going acid, which would stop the nitrification cold. The bacteria also feed on the carbonate and use it as a partial source of carbon. For every ten ppm of ammonia converted to nitrate very roughly 1 KH (18 ppm or dKH) of carbonate is used up.
4NH₃ + 4CaCO₃ + 7O₂ + 4CO₂ —> 4Ca(HCO₃)2 + 4HNO₃
During nitrification, 7.14 mg of alkalinity as CaCO₃ is destroyed for every milligram of ammonium ions oxidized. A lack of carbonate alkalinity will stop nitrification. In addition, nitrification is pH-sensitive and rates of nitrification will decline significantly at pH values below 6.8. Therefore, it is important to maintain adequate alkalinity in the aeration tank to provide pH stability and also to provide inorganic carbon for nitrifiers.
“How Alkalinity Affects Nitrification”, Barillo, 2015:
This means that one has to add considerable alkalinity to the aquarium water during cycling. It is best to add sodium carbonate until the pH hits 7.4 or higher every single day with fishless cycling. This means for every one gram ammonia salt (generally about 1/4 th ammonium) added one will very roughly two grams sodium carbonate.

Further Data
Then there is the science behind beneficial bacteria and cycling for those who are curious. This science is reviewed in the following links:
The topics of biofiltration, beneficial bacteria and cycling are closely related. So if one is interested in delving deep into the science and the calculations behind all aspects of biofiltration the following is pertinent:


