ORP stands for “Oxidation Reduction Potential” while Redox stands for “Reduction/Oxidation.” Both terms are interchangeable. Both refer to a process in which one chemical substance is reduced and another chemical substance is oxidized. They are defined as the measure in millivolts of the tendency of a solution of chemical substances to oxidize or reduce all the chemical substances in the solution.
Redox used by some individuals to gauge the health of an aquarium. These individuals seem quite sincere in their beliefs. Unfortunately the science is simply not that simple. Redox as a single number is not a good gauge of the health of an aquarium. Period. End of story.
If you are a real science nerd like the author and want to become very bored, read on.

Redox In Depth
Redox is used in the scientific community as a simple way to gauge the oxygen content of organic sediments. This is ALL it is used for by the scientific community.
Redox, at its heart, is extremely complicated. It is, perhaps, the single most complicated chemical feature of aquarium that aquarists will encounter. Redox in any aquarium involves many chemical details that are incredibly complex. It involves literally billions of processes that are not at equilibrium, and so are difficult to understand and predict.

All living creatures including bacteria are busily doing all sorts of reduction/oxidation reactions. These living creatures are the reason this is so complex. If one were looking at redox in a sealed container where the contents have been sterilized, then redox might have a far more relevant point. In water where there is no living creatures things become MUCH simpler.
So using a single number such as the redox to describe the condition of an aquarium and to diagnose problems in that aquarium is simply not possible. Redox is a weak indicator of the amount of aeration one has in the aquarium. In general, the higher the redox, the healthier the aquarium because generally the more aeration will be in place. But this is far from the full picture and very healthy aquariums can have low redox’s and unhealthy aquariums can have high redox’s.

Proponents of using ORP
One Facebook commentator commented on using water conditioners to remove chlorine:
All water conditioners use sodium thiosulfate to remove chlorine. Well….sodium thiosulfate is an oxidizer that lowers the redox potential in water chemistry from 400+mv to less than 300mv which any water under 280 mv is by scientific definition: sewage wastewater. So don’t use conditioners! You can maintain fish while using sodium thiosulfate, but it limits growth/ health potential. At my farm I run 560mv in all my tanks. Anyone who uses sodium thiosulfate to remove chlorine has a 330mv or even lower reading in their tank of redox. The redox determines and also indicates water chemistries health. If 420+ in good condition. If 380-419 this is bad and most likely will cause stress and health deterioration/ disease
This is one of those moments where things gets difficult to sugar coat or spin. This sincere individual is simply wrong. Sorry for my bluntness.
Sodium thiosulfate is a weak reducing agent. It ONLY reacts with strong oxidizing agents such as chlorine. It does not react with weak oxidizing agents like oxygen. Normally more sodium thiosulfate is added than that required for chlorine oxidation (we recommend using five times the recommended amount!). So it will not be oxidized completely in most aquariums when it is added.
This excess of a weak reducing agent will ONLY make the redox number go down. It does NOTHING else to the aquarium. This is completely harmless to fish. It DOES NOT remove the oxygen from the water, and it DOES NOT create conditions which are in any way detrimental to the fish.

American Aquarium Products or AAP (Carl Strohmeyer) is the major proponent of making redox (ORP) very important. On their 23 page JPG website on the subject (https://www.americanaquariumproducts.com/Redox_Potential.html) AAP says things like:
“So it is very important to keep a healthy Redox Balance via both sides of the Redox equation;
(a) Normal oxidizers such as proper/optimum dissolved oxygen levels.
(b) To counter oxidative stress (often artificially induced in our aquariums); via proper positively charged mineral levels (such as Calcium and Magnesium) and even level 1 or higher UV Sterilization.”
“Controlling oxidative stress such as the use on constant mineral replenishers & UVC, results in longer life spans of fish and higher disease resistance!!”
“PLEASE NOTE; many UV Sterilizers now marketed for under $50 CANNOT perform Redox reduction due to their poor dwell time, low cost medium pressure UV bulbs, and other factors, these are at best clarifiers and should NEVER be purchased unless that is all you desire from your UV!”
“These factors have the most affect in redox balance maintenance:
1, Water Changes; this is the most obvious and simple, however this is often not sufficient and sometimes the new water used does not have adequate mineral ions (especially if RO water is used even in part), so supplementing with mineral replenishers, such as SeaChem Replenish, Wonder Shells, etc. (even during water changes may be necessary).”
“Product Resources:
- Unique/Fresh AAP Wonder Shells
- Aquarium/Pond UV Sterilizers CAPABLE of improving Redox
- SeaChem Replenish
- AAP Redox/PH METER #H198121”
This website seems to be sincere about the use of Redox but their comments simply … well … , how do I diplomatically put this? … they just do not make a lot of sense. And then they have these underlined blue links to where one can buy these four products from AAP at inflated prices. This is a problem which introduces obvious biases. Is the author of this article genuinely confused or is he simply knowingly using pseudo-science to sell a product?

So AAP has 23 pages of obvious pseudoscience pushing redox as the cure for everything in the aquarium. Then they are selling AAP Redox Meter for an ungodly price ….. hhhmmmm … Is the pseudo-science simply genuine confusion or is AAP trying to sell a product?
AAP pushes the use of the high-priced UV units they stock to “optimize redox”. They have YouTube videos where they show the purple color of potassium permanganate is removed by their UV units. They then say that this proves these high priced UV units are beneficial in the aquarium.
This is simply wrong. Yes, UV reduces the permanganate ion. But that does not prove ANYTHING! And the high-priced units AAP say are necessary in a tank are NOT any “better” than the lower priced units available elsewhere. ANY UV unit removes the color in potassium permanganate. Then they are selling AAP UV light for an ungodly price ….. hhhmmmm … Is the pseudo-science simply genuine confusion or is AAP trying to sell a product?
AAP has YouTube videos where they use potassium permanganate to “prove” their “Wonder Shell” product is “beneficial” in the aquarium because it “maintains the redox balance”. The “Wonder Shell” product is probably just a small amount of sodium thiosulfate (which removes chlorine and chloramines in water change water) in a calcium carbonate/calcium sulfate (Plaster of Paris) matrix.
Sodium thiosulfate is a mild reducing agent. Purple colored potassium permanganate is a strong oxidizing agent which becomes colorless in the presence of even mild reducing agents. So the sodium thiosulfate in the Wonder Shell product removes the color in potassium permanganate.
Hhhhhmmmm …. OK, so what? Any and all water conditioners will remove the purple color. It proves nothing other than the product can be used to neutralize chlorine. Beyond the chlorine neutralization there is no “health benefit”. None. Then AAP is selling high priced blocks of Plaster of Paris ….. hhhmmmm … Is the pseudo-science simply genuine confusion or is AAP trying to sell a product?

To show how off the mark this AAP author is, one only need look at a chart of reducing and oxidizing potential shown in this article. The chart is used by AAP to “prove” how the “calcium” in its “Wonder Shell” product is beneficial to redox and to the aquarium. The chart (copied directly out of the article) is this:
: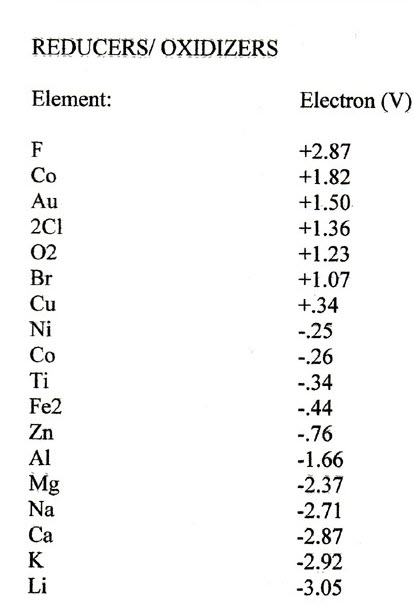
This chart is a correct chart for the electro voltage potential of each ELEMENT in its PURE ELEMENTAL FORM. In this chart the “Ca” is calcium and has a -2.87 electro voltage, which is a VERY strong reducing agent. The article than goes on how “Wonder Shells” add “calcium”, which is a “great reducing agent”, and obviously is very beneficial in the aquarium.
There is just one big problem. This chart is for the elements in their 100% pure form. In other words, “Ca” or calcium in the its “pure” or ELEMENTAL form is an extraordinarily strong reducing agent. Indeed, pure ELEMENTAL calcium must be kept under kerosene to keep it from oxidizing and burning in air. If ELEMENTAL calcium is put into water the reaction will be a rather violent, “exothermic”, rapid release of hydrogen gas which often just explodes.

But “Wonder Shell” is NOT elemental calcium. It is largely a chemical called calcium sulfate (also know as Plaster of Paris or gypsum). When calcium sulfate dissolves it produces calcium IONS. Calcium IONS are completely and totally different than ELEMENTAL calcium. Calcium IONS are NOT reducing agents in ANY way. So putting the above chart in this AAP article makes no sense what-so-ever.
So does the AAP author simply not understand basic chemistry or is he just trying to sell his AAP Redox meters, “Wonder Shells”, overpriced UV units and Seachem Replenish with pseudo-science? Who knows?
Note that while as I have pointed out Mother Nature can be very flexible and unpredictable in many areas related to fishkeeping, this isn’t one of those areas. The above is based on firmly-established, indisputable facts of chemistry.


